| SL.NO. | Authors | Title | Brief Description | Graphics |
|---|---|---|---|---|
| 1 | Shashadhar Samal, Editor, CSR |
The most polar nonionic aliphatic and aromatic compounds</ |
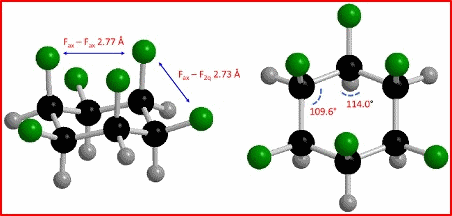
In April 2015, David O'Hagan, Neil S. Keddie, and coworkers (University of St. Andrews, UK) reported the synthesis of all-cis 1,2,3,4,5,6-hexafluorocyclohexane having a dipole moment of 6.2 D. The magnitude of molecular dipole moment is the highest for an aliphatic organic compound. In February 2016, Klaus Müllen and coworkers of the Max Planck Institute for Polymer Research made the world’s most polar aromatic compounds. Hexa-substituted benzenes with electron-withdrawing cyano groups and electron-donating amino groups align the molecule’s electron density in the same direction. The compounds are remarkable for being the most polar neutral molecules now known to exist with dipole moments of 10 D. |
|
| 2 | Shashadhar Samal, Editor, CSR |
Coordination number attained a theoretical maximum in clusters and Werner-type complexes |
In October 2015, Alexander I. Boldyrev of Utah State University, Lai-Sheng Wang of Brown University, and their colleagues reported cobalt-centered boron and fluorine cluster CoB16– having the highest coordination number known heretofore in chemistry. The drum-like structure is a molecular sandwich made of two B8 rings connected to the central cobalt atom via 16 bonds, the theoretical maximum based on the number of available atomic orbitals. In June 2016, Klaus-Richard Pörschke and coworkers of the Max Planck Institute reported Cs[H2NB2(C6F5)6], a Werner-type molecule in which 16 fluorine atoms coordinate a central Cs atom from five anion units. |

|
| 3 | Shashadhar Samal, Editor, CSR |
Largest Aromatic Nanoring |
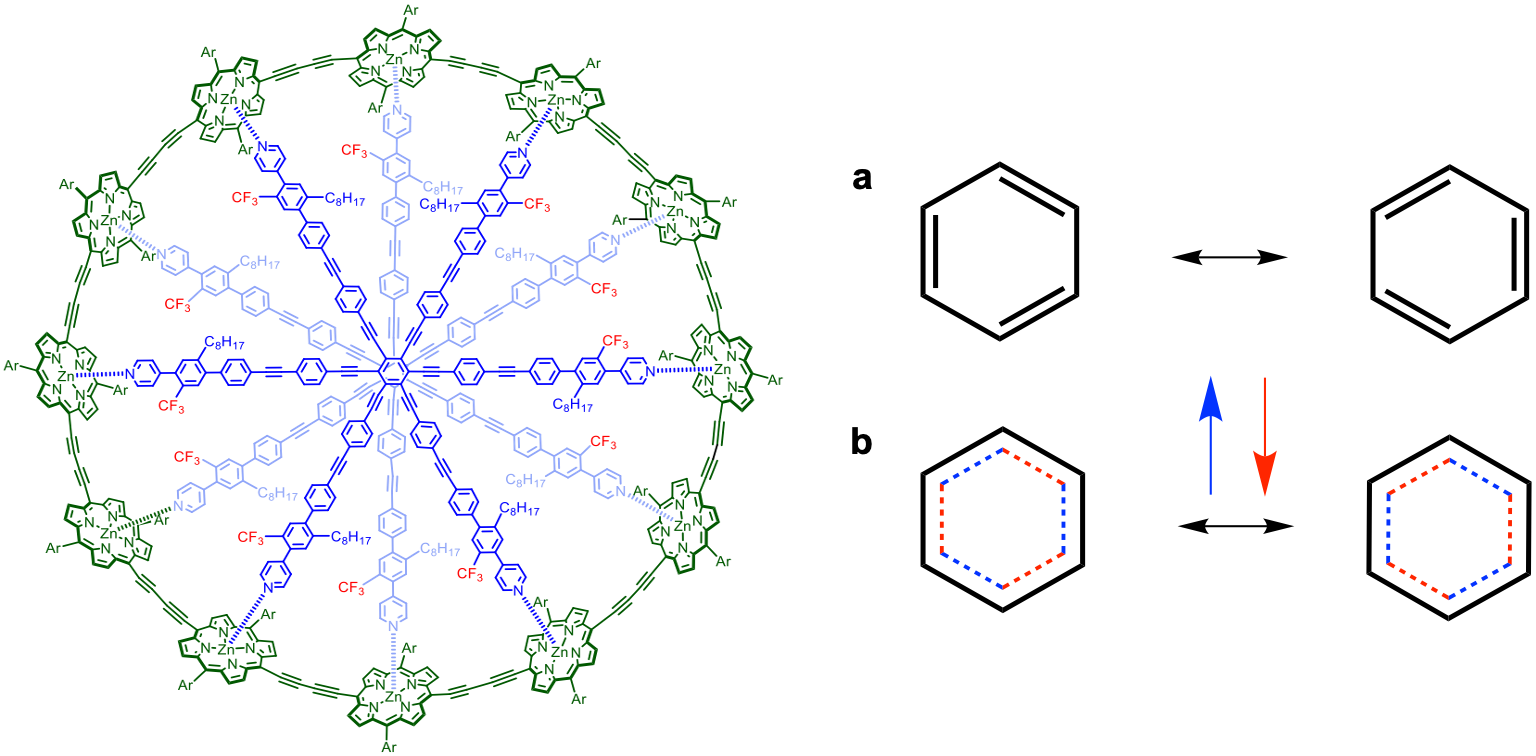
In January 2020, Harry L. Anderson and coworkers of Department of Chemistry, University of Oxford, UK, presented evidence for global aromaticity in porphyrin nanorings with up to 162 π-electrons (n = 40). The largest nanoring has a circumference of 16 nm and a diameter of 5.1 nm. The authors controlled aromaticity by changing the constitution, oxidation state, and conformation of the molecules. Hückel’s rule was formulated to explain the unusual properties of benzene and other molecules with 6 π-electrons. The simple rule correctly predicts the magnetic response of such large nanorings. In March 2020, Timothy W. Schmidt and coworkers of ARC Centre of Excellence in Exciton Science, School of Chemistry, UNSW Sydney, Australia, re-examined the structure of benzene from the principal effect of electron correlation. The authors concluded that electrons would not be spatially paired when it is energetically advantageous to avoid one another! This work underlines that a complete qualitative description of electronic structure, where resonance is exhibited, requires the unpairing of spins. Over 150 years after Kekulé proposed the structure of benzene, debates are still on as to what could be the actual structure of benzene.
|
|
| 4 |
Semantee Bhattacharya and Jyotirmayee Dash* |
Nucleic acid secondary structures for therapeutic and biomolecular device applications |
This review highlights the therapeutic targeting of G-quadruplexes and i-motifs by selective ligands endowed with potent biological activities. These novel molecular probes can selectively target DNA secondary structures over duplex DNA and promote cancer cell death. We also outline the development of advanced functional nanostructures like ion channels, bio-nanowires, logic gates, and enzyme-regulated DNA-based devices by conjugating G-quadruplex or i-motif with organic scaffolds. This review also focuses on the supramolecular chemistry of nucleic acid components to generate nucleoside-derived hydrogel networks and synthetic ion channels. These studies would provide new dimensions into anti-cancer therapeutics and nano-biotechnology using DNA secondary structures. |

|
| 5 | Abhijit Rana, Subhrakant Jena, and Himansu S. Biswal* |
Expanding the Frontiers of Non-covalent Interactions |
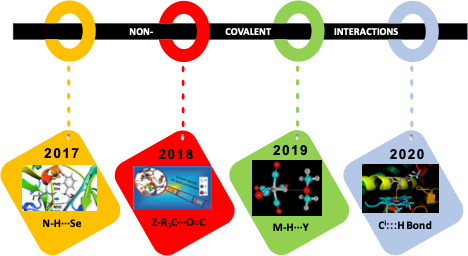
Non-covalent interactions are critical in maintaining the structural integrity of large molecules such as proteins, nucleic acids, and many supramolecular building blocks. One of such interaction is H-bond. Our group is always fascinated to understand the nature and strength of H-bonding interaction that involves sulfur and selenium despite their low electronegativity. The strength of such non-conventional H-bonds is very similar to that of their oxygen analogs. It is found that dispersion and polarizability play a crucial role in explaining such unusual behavior. Also to this, we have shown the existence of hydrogen bond in metal hydrides despite having electropositive metal center as H-bond donor. They also form strong H-bonds with S and Se and are dispersive in nature. Not only H-bond but also we looked at the ubiquitous presence of C-bond in proteins and its importance in myoglobin dissociation. More interestingly, we explored a bi-directional non-covalent interaction in the crystal structure of substituted propellane that arises due to synergistic contributions from C-bond and H-bond, and we named it a carbo-hydrogen bond. |
|
| 6 | Suchismita Subadini and Harekrushna Sahoo |
Fluorescence Spectroscopy: A Probe for Molecular Conformation and Dynamics |
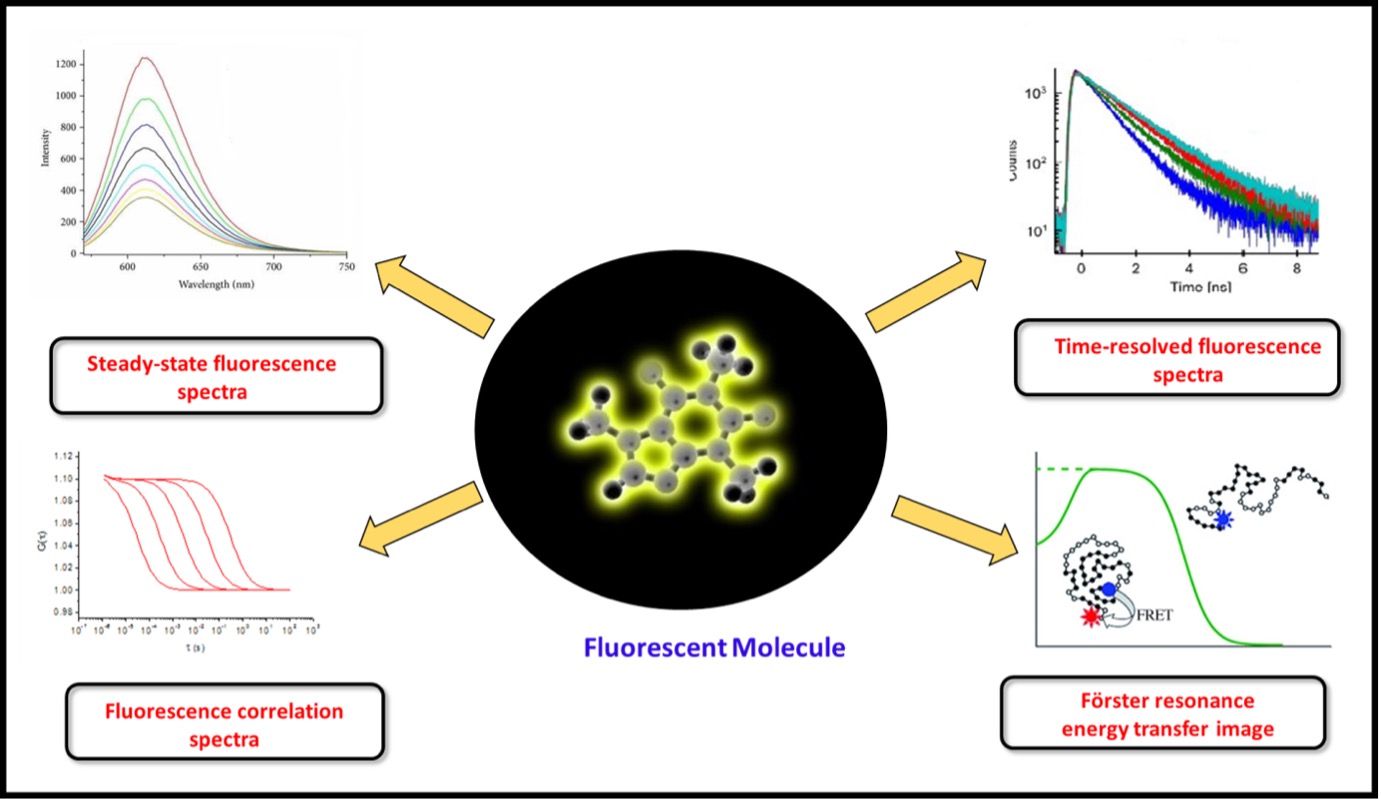
Fluorescence spectroscopic methods are growing rapidly because of their applications in diverse areas. Methods like steady-state and time-resolved fluorescence are most commonly explored to characterize emissive properties of fluorophores and their locale. Synchronous fluorescence spectroscopy (SFS) plays a significant role in analyzing multicomponent samples over conventional fluorescence spectroscopy. More importantly, among the methods, time-resolved outcomes contain more information about the kinetics of intra- and inter-molecular processes than that is not available from the steady-state. From the technique aspect, fluorescence anisotropy remains a popular tool to study biomolecular dynamics because of its sensitivity, robustness, independence of probe concentration, and simple instrumentation. Moreover, fluorescence anisotropy can be used to study many other biomolecular processes such as drug-protein and protein-protein interaction and protein aggregation. In contrast, the structural dynamics is extracted from fluorescence resonance energy transfer (FRET) data. More sophisticated techniques such as fluorescence correlation spectroscopy (FCS), very versatile and powerful in determining the dynamic behavior at a single molecular level, are based on time-averaging fluctuation analysis of fluorescence intensity generated in a tiny volume and can easily achieve single-molecule sensitivity. In addition to that, Fluorescence microscopes enable the imaging of various fluorescence probes inside the samples and help tremendously in obtaining fundamental insights into the structure and functions of biological systems. |
|
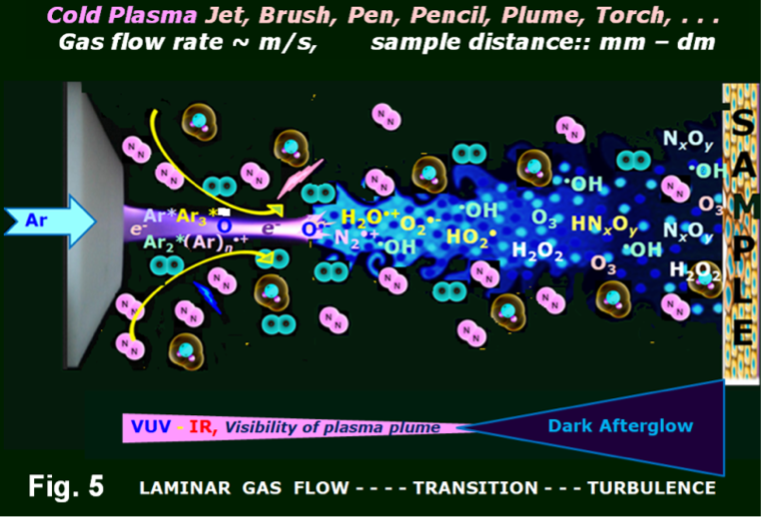
| 7 | Tomi Nath Das |
Mimicking Nuclear Radiation-Induced Chemistry with Friendly Cold Plasma |
Chemistry and related research that aims to address and solve myriad challenges is ultimately evaluated or judged from practical points of view. A successful endeavor generates a sense of satisfaction and opens new research directions; it may also substantiate the novelty and apt use of the experimental methodology chosen therein. Diversity in research strategies, aided by improvements and availability of related instrumental facilities, progressively allow more complex challenges, which seemed beyond reach previously, to be addressed and resolved. In this context, systematic estimations of any reaction’s physicochemical parameters and its underlying stepwise mechanism in any occurrence have always remained an essential attribute of research. Such quantifications allow user control of chemistry if desired and help alter or enhance productivity selectively by tinkering with a ‘reaction environment’. Chemical reactions’ commencements almost always necessitate energy input in some form and often set out either in the oxidative or reductive milieu. Nuclear Radiations (NR) such as high energy g- and X-rays (em radiation), and particles such as a,b, H+etc. from radioactive decay can create redox environments in a variety of media. Thus, for more than half a century, machines such as particle accelerators and gamma-chambers (in addition to non-nuclear sources such as LASERs and UV-vis light) were routinely deployed as sources for convenient and speedy evaluations of various types of reaction properties. Such measurements made the world over in different facilities and laboratories have played a pivotal role in enriching our shared knowledge base. In the last two decades, an exciting technology relating to Cold Atmospheric Plasma or Cold Plasma (CP in short), with its inclusive reactive energy, has emerged and matured rapidly. It offers an undemanding alternative to the NR studies that stipulate the use of vastly expensive and very complex hardware systems. Though the history of CP science and technology spans over the last 150 years, it remained utterly dormant or sparsely researched until the 1980s, mainly due to ignorance. At present, setting up a CP experimental facility or laboratory is an uncomplicated and generally an easy to finance proposition. Convenience in tuning CPgeneration currently makes it one of the user-friendliest technologies available for contribution in various chemistry-inclusive application areas, creating appropriate or innovative environments. Worldwide different laboratories, institutes, and even industries have found profitable use and application in diverse and utilitarian areas such as pollution control and mitigation, material surface modification, in biology, health and healing, nanomaterials and fabrications, polymers, improved vehicular engine performance, creating steps towards a cleaner and greener environment etc. to name a few. Herein, the “tricks of the CP trade” and some of its recent diverse multi-phase, atypical or innovative chemical and other applications, including some carried out in our laboratory, are presented to sensitize and motivate researchers about this novel topic for endless exploration. |
|
| 8 | Author |
Title |
Description |
|